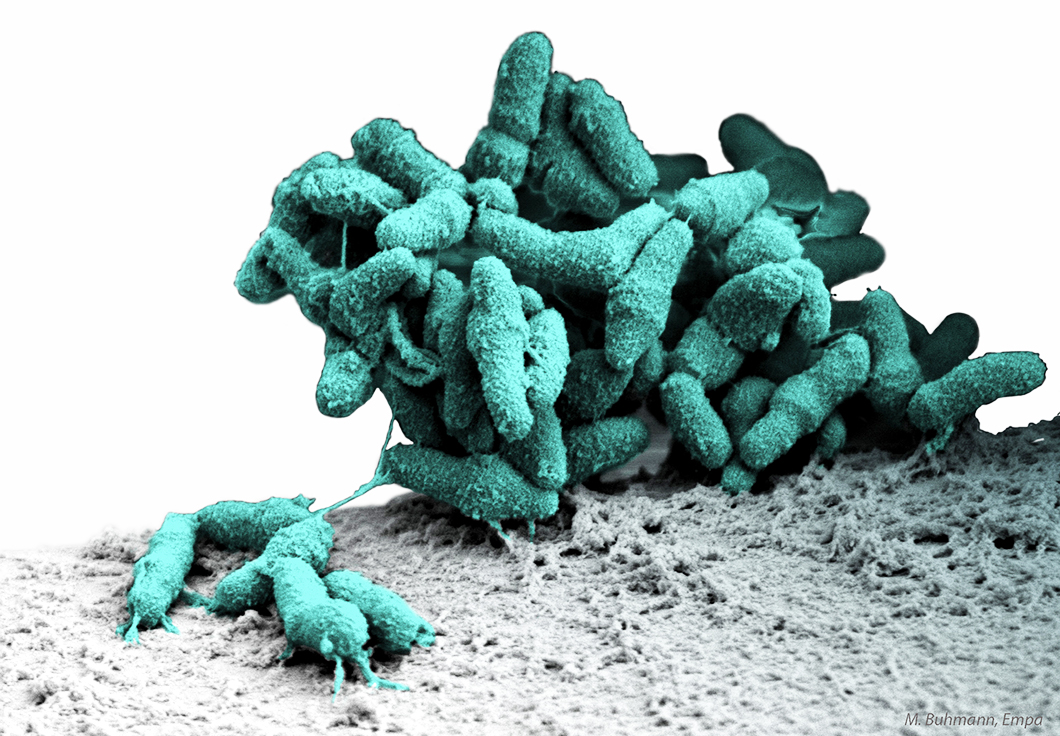
Bacteria growing as a biofilm on the surface of a ureteral stent
Source: Matthias Buhmann, EMPA
Project period
01/10/2017 - 30/06/2021
Project type
Collaborative research project
Project status
Closed
Description
The BEAT-AMR project is a partnership against biofilm-associated expression, acquisition and transmission of antimicrobial resistance.
Location
Bundesanstalt für Materialforschung und -prüfung
Unter den Eichen 87
12205 Berlin
Pseudomonas aeruginosa cells densely packed in a biofilm Source: BAM
In medicine, antimicrobial compounds are used as antibiotics or as coatings on biomedical surfaces such as implants or catheters. These surfaces, once inserted into the human body, can get colonized with bacteria forming a hard-to-treat biofilm. In addition, the recent decade has seen a dramatic rise of microbes that are resistant against multiple antimicrobial compounds. Currently, our knowledge about antimicrobial resistance (AMR) development in bacteria growing on biomedical surfaces is limited. In particular, the differences between resistant and non-resistant bacteria in the response to surfaces coated with antimicrobial compounds is not well understood.
Source: BAM
The work of our international consortium relies on a trans-European research infrastructure of microbiology, material science, and clinical research laboratories. Fundamental processes will be investigated in laboratory studies with the common biofilm-forming pathogen Pseudomonas aeruginosa. Biofilms will be grown on test surfaces and investigated with imaging technologies, such as atomic force microscopy and confocal laser scanning microscopy, and with modern sequencing technologies including metagenomics and metatranscriptomics.
Source: BAM
The aim of the project is to establish a fundamental understanding of the interplay between antimicrobial surface coatings, microbial biofilm formation, and antimicrobial resistance (AMR) evolution.
The consortium will use the acquired fundamental knowledge to test specific hypotheses directly in clinical settings at the final stage of the project. This approach will enable us to formulate clinical recommendations for the combined use of antimicrobial surfaces and antibiotics that minimize resistance spread and evolution.
Source: BAM
BEAT-AMR is a cooperation of four European partners: Bundesanstalt für Materialforschung und -prüfung ( BAM, Germany), Swiss Federal Laboratories for Materials Science and Technology (EMPA, Switzerland), University of Southampton (UK), University Medical Center Groningen (The Netherlands).
Budget: ca. 1.3 Mio. € from the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR).
The German Ministry for Science and Education (BMBF) finances the works conducted at BAM.
BEAT-AMR - Partnership against biofilm-associated expression, acquisition and transmission of antimicrobial resistance
Antimicrobials are used as antibiotics in medicine to fight microbial infections or as biocides to, for example, preserve materials and disinfect surfaces. The toxicity of antimicrobial compounds to microorganisms creates a strong potential for evolution of resistance. Antimicrobial resistance (AMR) is defined as the ability of a microorganism to grow in the presence of high concentrations of toxic compounds that are sufficient to kill non-resistant members of the same species. The emergence of AMR poses a major threat to modern health care systems by compromising our ability to control microorganism.
Biofilms are microorganisms living on surfaces that become embedded within a self-produced, extracellular matrix composed of polymeric substances like long-chained sugars, proteins or DNA. Biofilms can form on tissues or on biomedical surfaces, such as blood catheters or implants, where they act as a reservoir of potential healthcare-associated infections. In fact, it has been recognized that biofilms underlie the majority of chronic bacterial infections.
Dr. Frank Schreiber with bacterial cultures Source: BAM
Population dynamics during biofilm formation on antimicrobial surfaces
Objective: Identify antimicrobial-antibiotic combinations that select for and against antibiotic resistance and determine population dynamics of resistant strains in biofilms.
Schematic representation of bacteria, shown as rods, in a biofilm (detail) Source: Source: Jules Valentin, EMPA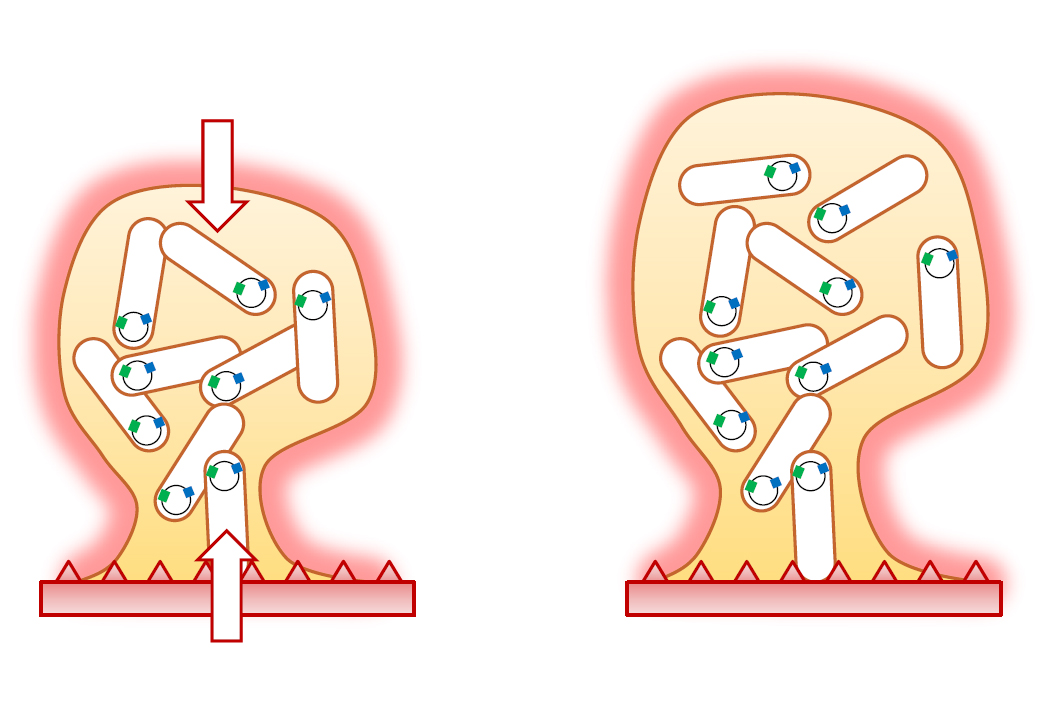
Genes involved in phenotypic adaptation to antimicrobials on surfaces
Objective: Identify genes that are involved in phenotypic adaptation against antimicrobials in biofilms.
Microfluidic device (detail) Source: University Medical Center Groningen

Biophysical properties of bacteria growing on antimicrobial surfaces
Objective: Determine the functions of the identified genes and of known AMR genes with regard to adhesion strength and whole biofilm biophysical properties, including transport of antimicrobials in biofilms.
Contribution of the University Medical Center Groningen to BEAT AMR
Cells growing on a surface as a biofilm Source: Odel Soren, University of Southampton
Genomic evolution in biofilms upon antimicrobial stress
Objective: Identify genes under positive selection within biofilms formed on biomaterials.
Bacteria living in biofilms can tolerate much higher antibiotic concentrations compared to planktonic bacteria and survive long enough to acquire AMR by changing their genetic code via mutations. They form persistent, hard-to-treat infections and exhibit an intrinsic biology that promotes the development and transmission of AMR. Preventive and therapeutic strategies against biofilm infections in clinical settings commonly involve the application of multiple antimicrobials. In addition to the implanted biomaterials being equipped with antimicrobial coatings, antibiotics are systemically administered to patients simultaneously.
The goal of the consortium is to determine how bacteria adapt to antimicrobials during biofilm formation on surfaces, how AMR mutations are acquired and evolve within mature biofilms, and how population dynamics within biofilms affect the transmission of AMR in multi-drug environments.
Funding
The work is funded within the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR).


