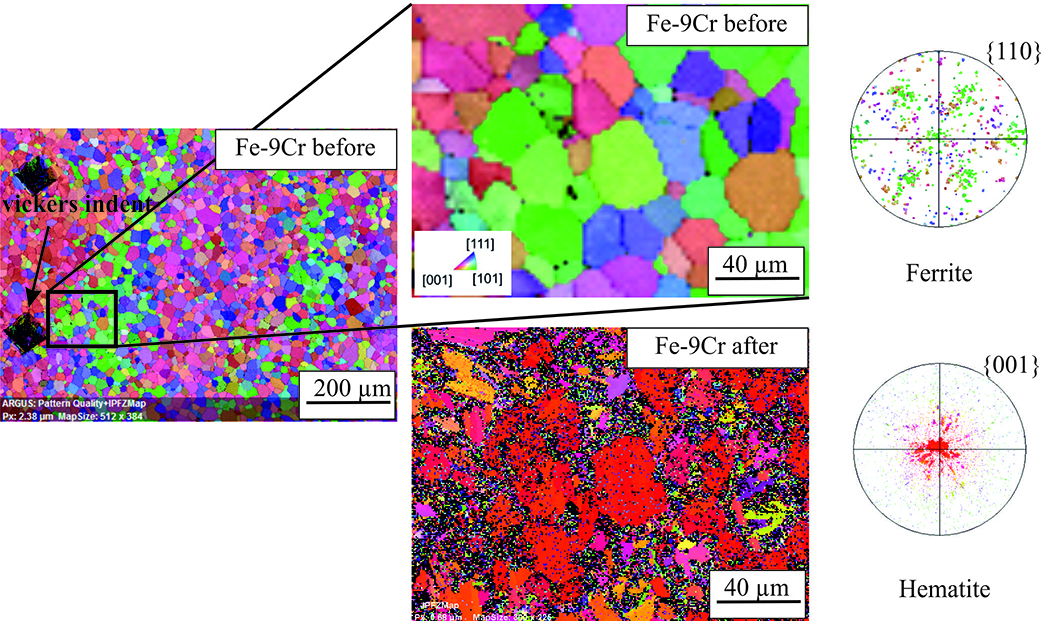
Orientation distribution map for Fe with 9% chromium before and after exposure to 0.5% SO2 at 650°C. The right side shows the pole figures for ferrite (Fe) and hematite (Fe2O3) according to the defined crystal orientation relationship.
Source: BAM, division Materialography, Fractography and Ageing of Engineered Materials
Polymer-based nanocomposites are new innovative materials, which consist of a polymeric matrix and an inorganic filler material, where at least one spatial dimension of the nanoparticles is below 100 nm. Polymer-based nanocomposites have a broad range of applications as light-weight construction materials for instance in automotive and aerospace industries, as well as in astronautics. Because the sizes of the nanoparticles are in the nanometer scale there is a high volume or weight fraction of an interphase between the nanoparticles and the polymeric matrix. The polymer segments in this interphase have different properties than those of the matrix. Due to the high volume fraction of the interphase the properties of the whole nanocomposite are codetermined by this interphase. Here a model system is considered, based on an anhydride crosslinked epoxy resin as matrix and Boehmite (oxide hydroxide of aluminum) as nanofiller which is investigated in detail by a combination of wide and small angle X-ray scattering, “advanced” calorimetry, as well as by broadband dielectric spectroscopy. With X-ray scattering, employing a Monte Carlo algorithm in combination with a model function for the scattering of the filler, it was possible to estimate the dispersion of the nanoparticles in the nanocomposite. Three relevant fractions of nanoparticles have been identified: Primary particles with sizes smaller than 30 nm as the main fraction, nanodomains with a size between 30 nm and 1 m and aggregates with a size larger than 1 m (fraction is smaller than 1 weight percent). The dependence of these fraction on the concentration of the filled-in nanoparticles is discussed in greater detail. With temperature modulated calorimetry it was possible to estimate the mass fraction of polymer segments adsorbed on the nanoparticles (interphase). For the first time a simultaneous mobilization and immobilization of segments in this interphase is found. A detail analysis of the data obtained by broadband dielectric spectroscopy reveals that the molecular dynamics is intrinsically heterogeneous. This indicates that the structure of the epoxy matrix is intrinsically heterogenous as well. The conclusion is supported in part by the X-ray data.
Competition of nanoparticle-induced mobilization and immobilization effects on segmental dynamics of an epoxy-based nanocomposite
Paulina Szymoniak, Brian Richard Pauw, Xintong Qu, Andreas Schönhals
published in Soft Matter, Vol. 16, issue 23, pages 5406 - 5421, 2020
BAM, Synthesis and Scattering of Nanostructured Materials division
und Physical and Chemical Analysis of Polymers division


