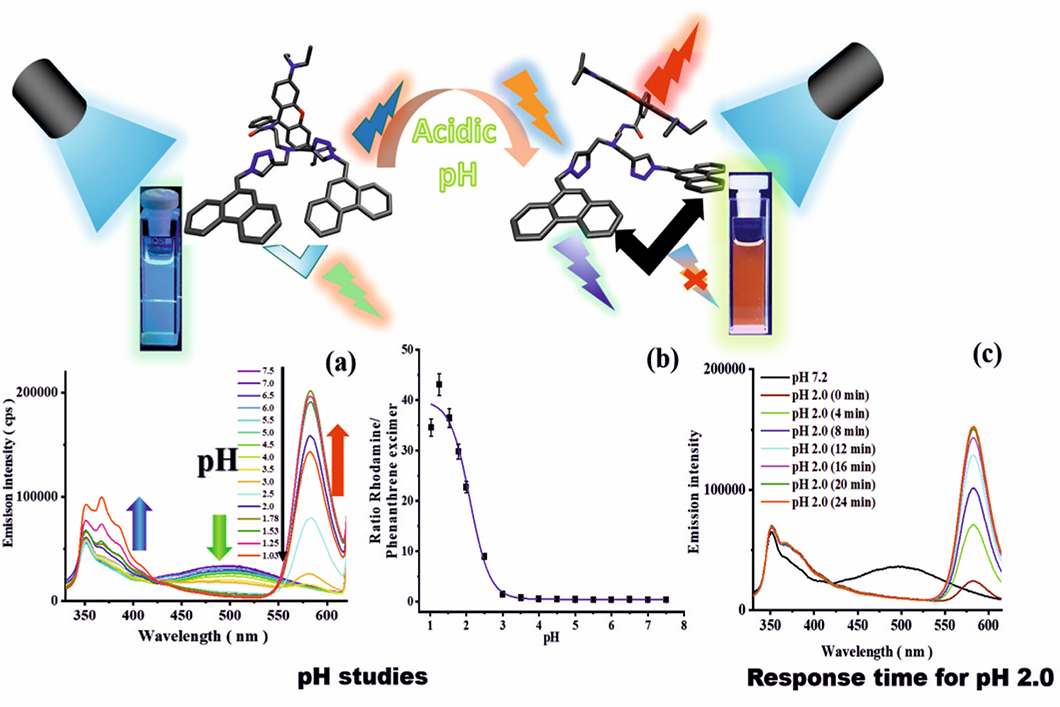
Top: Molecular structures of the sensor and color emitted at pH 7.0 and 2.0; bottom: (a) Emission spectra, (b) pKa plot, and (c) response time.
Source: BAM, Biophotonics division
pH, the negative logarithm of the proton concentration, is an often used parameter in the life and material sciences to indicate the proper functioning of cells, as a marker for diseases like inflammation and cancer, and as a hint for the occurrence of corrosion processes. pH can be measured optically with the aid of pH-responsive sensor molecules that change their absorption and/or emission color as a function of pH. Performance parameters of such sensor dyes, that can be used as stand-alone systems or integrated into supports such as polymer films or nanoparticles, include the size of the measured pH-induced optical changes, the absorption and emission efficiency, and the response time. To make the fluorescence-based analyte quantification reliable and independent of the excitation light intensity, that can fluctuate, a sensor molecule is often combined with a spectrally distinguishable analyte-inert reference dye in a so-called ratiometric sensor system. Subsequently, the quotient of the emission of both molecules is read out. To simplify the design of such systems, analyte-responsive molecules with a built-in reference are needed that emit two or even three colors. Currently, different dual and tricolor fluorescent sensor molecules are being developed in the division Biophotonics by integrating two or three dyes with different analyte responsivities into a single molecule. By combining two phenanthrene units with one rhodamine unit in one molecule, we obtained a tricolor emissive fluorescent sensor with emission bands at 351 nm (ultraviolet), 500 nm (green), and 582 nm (red). These bands originate from the phenanthrene monomer, the phenanthrene excimer formed by the interaction of two neighboring phenanthrenes in the excited state, and the rhodamine. The green phenanthrene excimer emission acts as a reference for pH measurements with the pH-responsive rhodamine, the red fluorescence of which is switched on upon protonation (pH-induced opening of the rhodamine´s spirolactam ring). The short wavelength emission of the phenanthrene monomer at 351 nm can also be exploited for pH studies. The excimer/monomer emission of phenanthrene and the emission of rhodamine can be used in combination or separately to precisely detect the pH of the environment of the sensor molecule over a broad pH range from 7.5 to 1.
Synthesis and spectroscopic characterization of a fluorescent phenanthrene-rhodamine dyad for ratiometric measurements of acid pH values
Priyanka Srivastava, Paul Christian Fürstenwerth, J. F. Witte, Ute Resch-Genger
published in New Journal of Chemistry, Band 45, Heft 31, Seite 13755-13762
BAM, Division Biophotonics


