Iron reducing bacteria fit for corrosion – An in situ electrochemical XANES study: Schematics of the measurement cell and processes
Source: BAM, Division Interfacial Processes and Corrosion
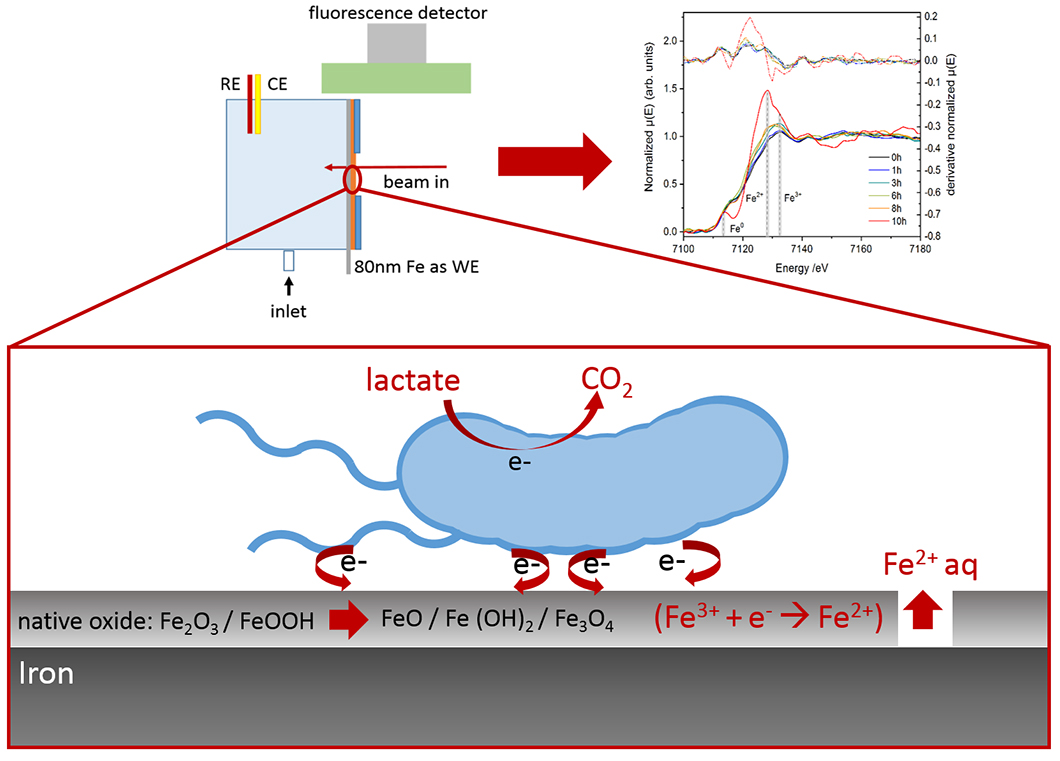
Electroactive facultative anaerobic bacteria as Shewanella putrefaciens CN32 are able to utilize various metal compounds as electron acceptors. These compounds include species found in the outermost oxide films, also called passive film, which protect steels from environmentally influenced corrosion processes. An alteration in the oxidation state of species within the passive layer can lead to the destruction of the protective effect and induce the onset of corrosion. This so called microbiologically influenced corrosion (MIC) is widely discussed in literature and researchers from all fields are searching for the best methods to study the electron transfer processes with an interdisciplinary approach. It is crucial to combine techniques that allow the in situ investigation of this bacterial attack for being able to elucidate the underlying processes without the re-passivation of the metal during drying processes or destroying the fragile biofilm interface.
In this paper a newly designed cell is introduced, that allows the investigation of corrosion processes on model iron thin films in the presence of metal reducing bacteria. The cell design enables the measurement of electrochemical processes at the metal-biofilm-electrolyte system as well as the simultaneous spectroscopic analysis of the metal oxidation states via X-ray absorption near-edge spectroscopy (XANES). The study presents the successful implementation of the cell for the in situ electrochemical XANES technique as well as our results on the effect of different growth conditions of the bacteria. The electrochemical activity of two cultures grown in the presence and absence of Fe(III) ions in the culture medium were investigated.
Both, XANES spectra and the open circuit potential data of the iron film incubated with the culture that was grown in absence of Fe(III) did not show any significant changes during twenty hours of monitoring. Whereas in the case of the culture grown in Fe(III) containing medium, an accelerated dissolution of the metal was observed accompanied by the conversion of the native oxides to a mixed Fe(II)-Fe(III) hydroxide surface layer. The open circuit potential steadily approached the free corrosion potential of iron in neutral chloride containing electrolytes, indicating a continuous dissolution process without passivation.
Trained to corrode: Cultivation in the presence of Fe(III) increases the electrochemical activity of iron reducing bacteria – An in situ electrochemical XANES study
Nina Wurzler, J. D. Schütter, R. Wagner, M. Dimper, D. Lützenkirchen-Hecht, Özlem Özcan
published in Electrochemistry Communications, Band 112, Seite 106673 ff.
BAM, Division Interfacial Processes and Corrosion


